What is a UDI, the Unique Device Identifier?
What is a UDI, the Unique Device Identifer?
The FDA has established and implemented a unique device identification system that is used to adequately to identify medical devices through their distribution and use. With these unique device identifers implemented, it will be able to trace and track medical devices, improve patient safety, modernize device post-market surveillance, and facilitate medical device innovation.
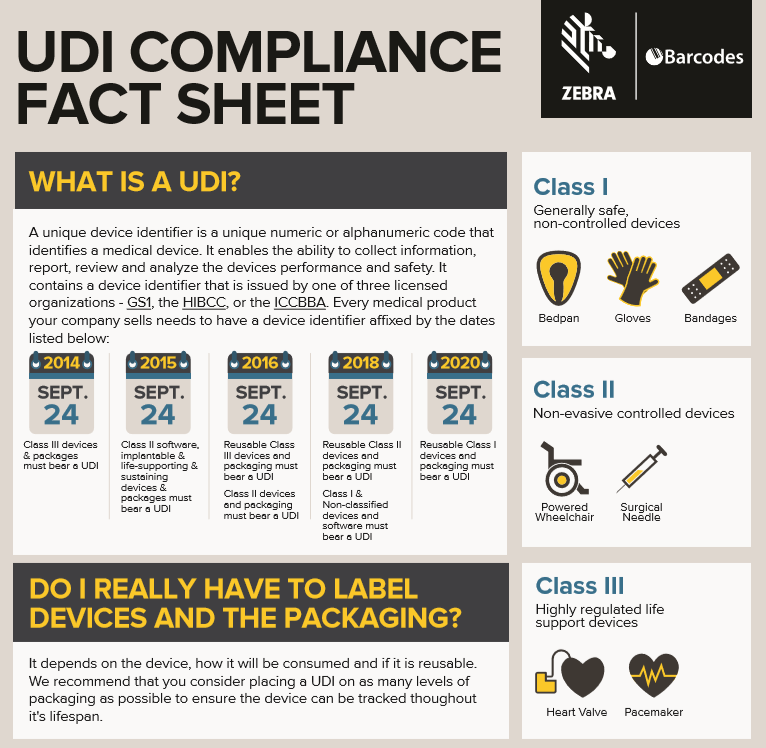
Currently, Class I,II,III medical devices distributed in the United States must carry a UDI to meet the requirements of the Food and Drug Administration(FDA). No UDI? No Business! Medical device products identification labels help maintain compliance, ensure brand consistency, improve operational efficiency, and support business growth.
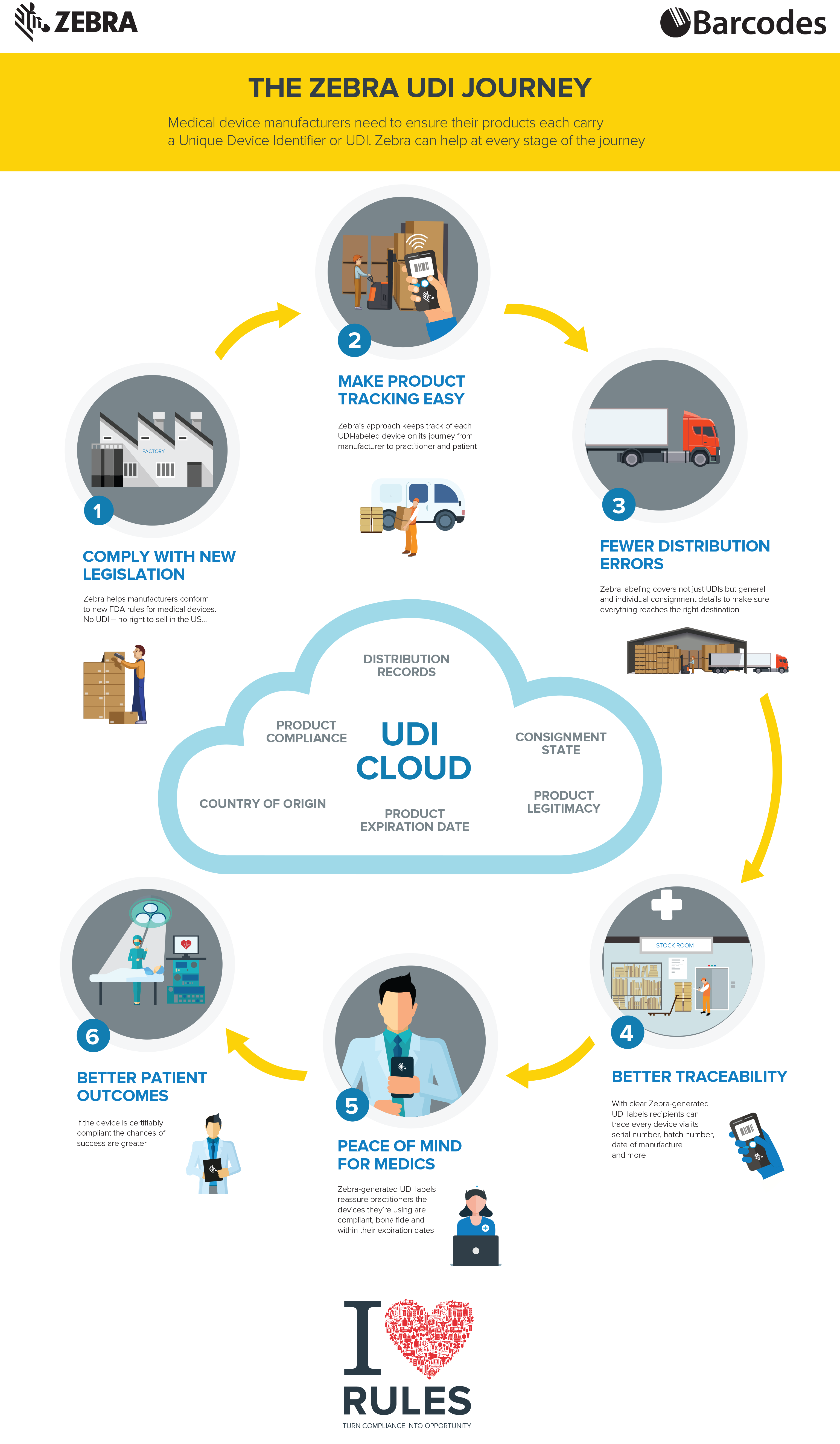
Check out the infographic and call us today to see how Barcodes and Zebra can help you at every stage of the route to become UDI compliant.
Currently, enjoy free 2-day shipping on all orders of $250+ made online at Barcodesinc.com through January 9,2019. Exclusions apply.
Enjoy free 2-day shipping on all orders of $250+ made online only at Barcodesinc.com through January 9, 2019 at 11:59 pm CST. Offer cannot be combined with other promotions or discounts. Offer valid on web pricing only. Offer valid in the US only. This offer is not valid on international orders. Certain product exclusions apply on supplies (ribbons/labels) and cash drawers.
The Guide to Succeeding with UDI Compliance
To go along with yesterday’s blog about Unique Device Identification Compliance some may be wondering what is required to be fully compliant with the FDA. With another UDI deadline approaching it’s important to know the steps that will help you get in compliance. With the help of Zebra Technologies, they wrote a small walk through to help you through each step.
Read the walk through below to clear up any confusion. If you have any further questions please don’t hesitate to contact our dedicated account managers.

Unique Device Identification Compliance
The Food and Drug Administration (FDA) has established and continues to implement a unique device identification system to properly identify medical devices through their distribution and use. Once fully implemented, the label of most devices will include a unique device identifier (UDI) in human and machine-readable form.
The unique device identification system will be phased in over the next couple of years and offers several benefits. Implementing UDI will improve patient safety, modernize device post-market surveillance, and facilitate medical device innovation. To learn more about UDI read the infographic below!
If you want to learn more, feel free to contact our dedicated account manager.
Barcodes, Inc. Delivers and Astute Solution
 Challenge
Challenge
Astute Medical manufactures biomarkers that are used to identify high-risk, acute medical conditions in critically ill patients. The Federal Drug Administration (FDA) issued a mandate that medical devices must carry a Unique Device Identifier (UDI), which requires a greater volume of information than had been previously required.
Astute Medical sought a compliance labeling solution that would meet UDI requirements in accordance with GS1 Standards. The manufacturer also wanted to receive accurate and repeatable verification results and a solution that included a Validation Protocol to document compliance with these requirements. We needed to not only comply with federal regulations, but also ensure that we were sending out our medical devices with a high-quality barcode, “to avoid returns,” explained Susan Shelton, Materials Management and Procurement Manager of Astute Medical.
Analysis
Astute Medical designs, engineers, and manufactures novel biomarkers that enable clinicians to identify acute conditions in their critically ill patients before the symptoms become evident. Products like the NephroCheck Test and Astute140 Meter fall under the guidelines of the FDA’s Unique Device Identification rule. This system was established to identify medical devices throughout their distribution and use, providing critical traceability that benefits the patient, caregiver, manufacturer, and distributor.
FDA Food Traceability with Zebra Labels
Ever since the FDA passed the Food Safety Modernization Act, anyone involved in the food supply chain has to be able to produce, at any moment, the movement of food from supplier to the final retail store. With legislation forcing food grower, manufacturers, and distributors to track food from field to the dinner table, having capable and affordable traceability technology is essential for everyone involved.
The biggest motivation behind the legislation is to make it easier to trace and find sources of contaminated food to the correct original source. This may seem at first as a measure mainly for the end consumer but it also protect growers and manufactures in being to very precisely remove their goods from suspicion of tainting as opposed to wide spread recalls. The costs involved in a capable barcode tracking system is noticeable less than even just one recall incident.
The Food Safety Modernization Act calls for, among other things, unique identifiers such as the GS1 databar, and full electronic pedigree or origins of the produce. Barcoding technologies are an important first step in this compliance as growers begin to change and update their record keeping methods. This is where Zebra and their wide range of printers and FDA approved labels are the perfect complete solution.
With printer options for low volume small businesses, GK420 and TLP2824 Plus, to high speed/high output models, ZT400 and 110Xi4, Zebra’s long standing industry experience and expertise is designed in every printer they make. From labeling a single tomato to whole crates and packaged foods, Zebra has a traceability solution that can improve your business’s accuracy and efficiency.
Zebra Labels for Indirect Food Contact FDA 175.105
 Many food packaging and repackaging businesses have a need to find a packaging label that has an adhesive suitable for indirect food contact. Most importantly these business need a labeling solution that meets Food and Drug Administration (FDA) guidelines for indirect food contact. Indirect food contact is defined as a substance that may come into interaction with food as part of packaging or processing equipment, but are not intentionally added directly to the food. Using a label that doesn’t meet food contact requirements can potentially lead to contaminated food, product returns and fines for having unapproved adhesives used in a food application.
Many food packaging and repackaging businesses have a need to find a packaging label that has an adhesive suitable for indirect food contact. Most importantly these business need a labeling solution that meets Food and Drug Administration (FDA) guidelines for indirect food contact. Indirect food contact is defined as a substance that may come into interaction with food as part of packaging or processing equipment, but are not intentionally added directly to the food. Using a label that doesn’t meet food contact requirements can potentially lead to contaminated food, product returns and fines for having unapproved adhesives used in a food application.
Zebra manufactures several sizes and types of labels that have an adhesive formulation developed to meet FDA regulation 175.105, indirect food contact (see this list for FDA approved labels). These labels are approved to be used for indirect contact food applications such as, label food in a retail location to large scale packaging of food from a manufacturing facility.
Avoid the hassle and expense of using non-food approved labels by introducing Zebra labels, which meet FDA regulation 175.105 for indirect food contact, into your food application today.
Contact your dedicated BarcodesInc representative today, for more information.
TEKLYNX Now Supports New Food Manufacturing Label Requirements

TEKLYNX continues to support manufacturers as they address industry and regulatory changes. Making headlines recently are three food manufacturing initiatives that will affect the way suppliers create labels: the Produce Traceability Initiative (PTI), the Food Information for Consumers regulation (FIC) and proposed FDA changes to Nutrition Facts labels. TEKLYNX barcode labeling software supports the creation of label designs for all three initiatives.