Unique Device Identification Compliance
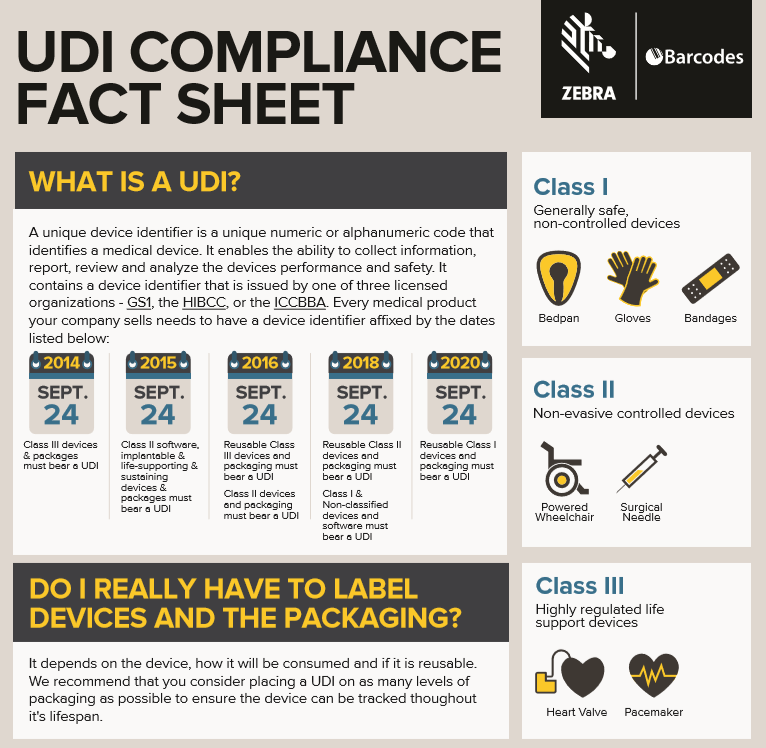
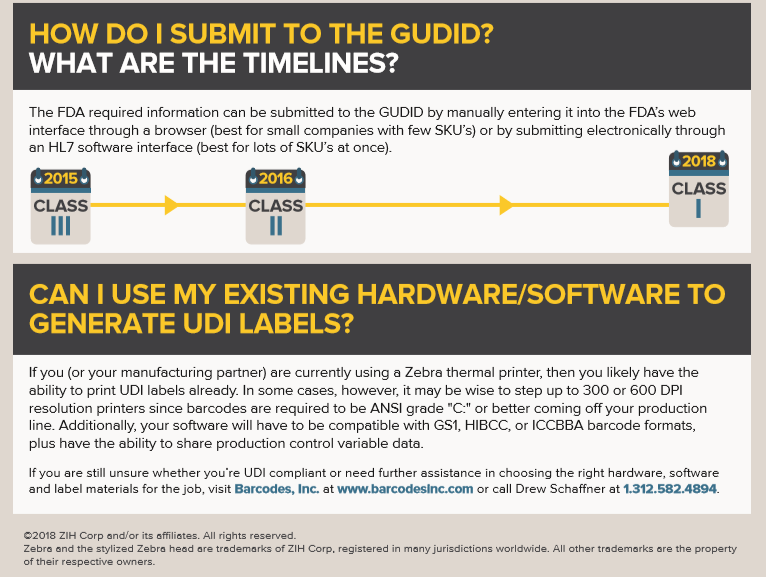
The Food and Drug Administration (FDA) has established and continues to implement a unique device identification system to properly identify medical devices through their distribution and use. Once fully implemented, the label of most devices will include a unique device identifier (UDI) in human and machine-readable form.
The unique device identification system will be phased in over the next couple of years and offers several benefits. Implementing UDI will improve patient safety, modernize device post-market surveillance, and facilitate medical device innovation. To learn more about UDI read the infographic below!
If you want to learn more, feel free to contact our dedicated account manager.