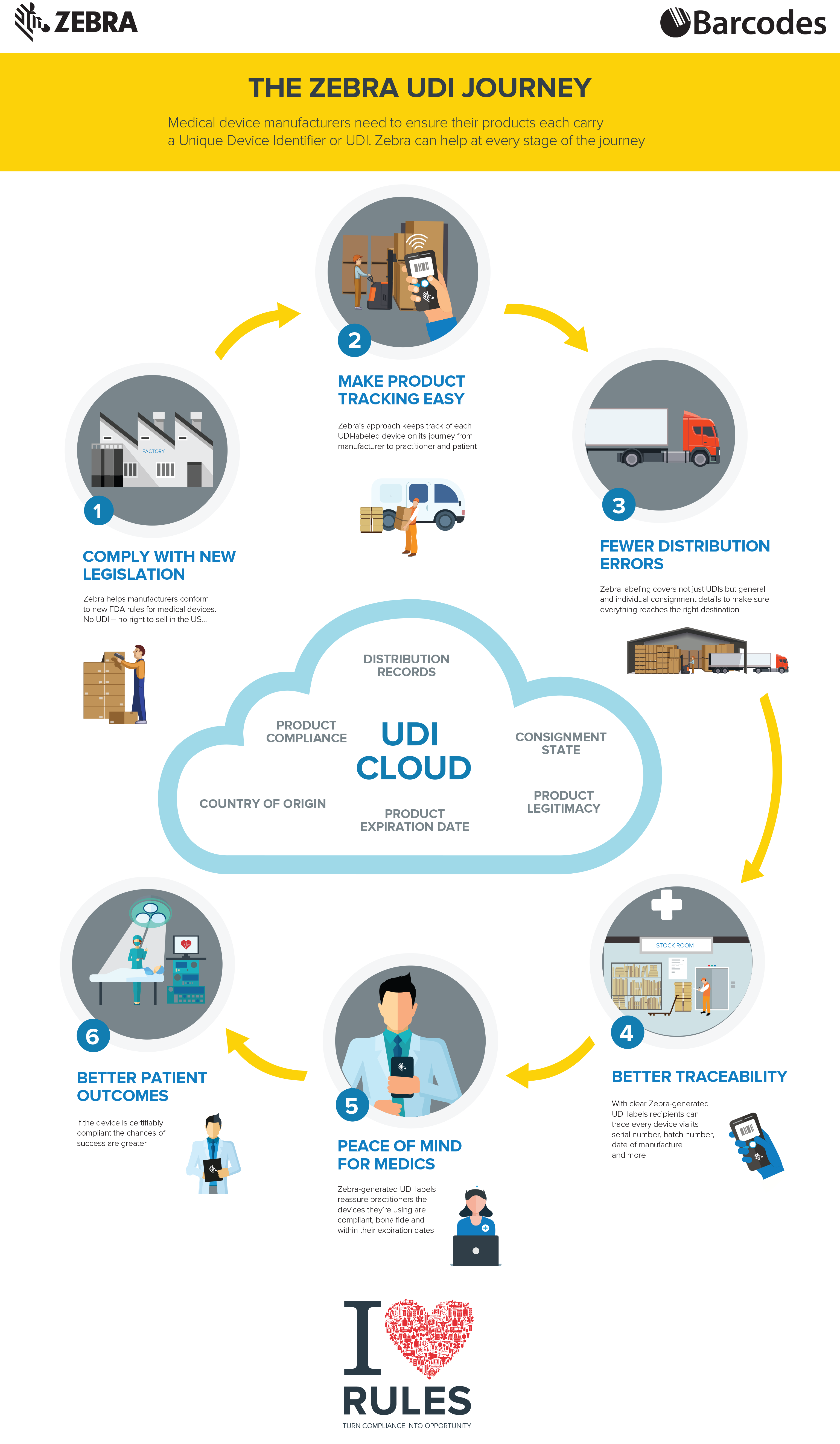
What is a UDI, the Unique Device Identifier?
What is a UDI, the Unique Device Identifer?
The FDA has established and implemented a unique device identification system that is used to adequately to identify medical devices through their distribution and use. With these unique device identifers implemented, it will be able to trace and track medical devices, improve patient safety, modernize device post-market surveillance, and facilitate medical device innovation.
Currently, Class I,II,III medical devices distributed in the United States must carry a UDI to meet the requirements of the Food and Drug Administration(FDA). No UDI? No Business! Medical device products identification labels help maintain compliance, ensure brand consistency, improve operational efficiency, and support business growth.
Check out the infographic and call us today to see how Barcodes and Zebra can help you at every stage of the route to become UDI compliant.
Currently, enjoy free 2-day shipping on all orders of $250+ made online at Barcodesinc.com through January 9,2019. Exclusions apply.